The seller claims it can be excited by heat, but this is of course nonsense. In general only wavelengths shorter than the emission spectrum can excite so infrared and heat can only expedite the release of the already stored energy, but not cause excitations. Excitation by heat under equilibrium condition is forbidden by the laws of thermodynamics.
It it is still true that under nonequilibrium conditions such as high intensity infrared irradiation can a process called frequency upconversion occur, but these are very rare events, do not apply to this case and do not come into conflict with the laws of thermodynamics.
I made a quick detector from a photomultiplier tube and a metal can and the proceeded to log the tube output using a raspberry pi and a digital multimeter.
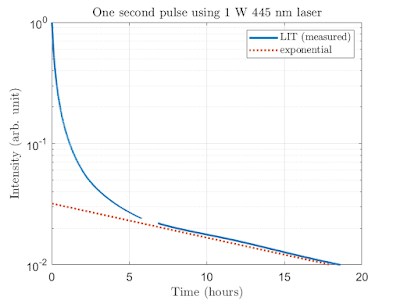
It seems this material doesn't exhibit typical exponential decay one would normally expect, but rather it seems to decay first at a faster rate and then slow down. The measurements below indicate that the intensity will drop to approximately 1:10 of the initial intensity during the first hour, but will take something like 18 hours to drop to 1:100. Human eye adjusted to dark can still detect the output probably even if it's only 1:1000 of the initial. I don't know what exactly is the cause of this non-exponential behavior in this material, but I suspect there is some kind of statistical mix of different decay times at play here. Unfortunately I couldn't find my strontium aluminate right now for comparision. I guess some events are fast, some are slow and these then together will lead to this decay curve. I know I'm far from the dark counts of the tube and it's not saturating either so all should be good. The quantitative results also seem to match the qualitative results as far as my eye can tell so this really is the way this material behaves. Thiugh, after long enough it seems to settle to exponential decay so perhaps the assumption is correct. Perhaps I should also check how the emission spectrum looks like, but for that I'll have to wait for my spectrometer to arrive.
This is the same detector which can count individual photons. Although, the quantum efficiency is only slightly above 20%.
...got my spectrometer now and measured a bunch of things...
Monitor red doesn't appear to be very natural. Because I excited quinine with 404 nm laser, the peak is seen in the fluorescence spectrum as well as fluorescence (fluorescence lifetime is so short that afterglow can't be measured directly this way). LIT phosphorescence spectrum doesn't show the 445 nm excitation as the excitation is off when collecting the spectrum as phosphorescence afterglow decays slow enough. My 445 nm laser seems to be frequency upconverted from IR and the IR peak is visible as well (barely at the edge of the range for my spectrometer).
| Spectrum lines of my monitor displaying all white image |